The heat capacity of aluminum at room temperature (25 °C) is approximately 0.897 J·g⁻¹·K⁻¹, which means that to raise the temperature of 1 gram of pure aluminum by 1 kelvin, roughly 0.897 joules of heat energy are required. This relatively high heat capacity makes aluminum an excellent material for applications demanding efficient thermal buffering and energy storage.
1. Atomic Origins: Why Aluminum Absorbs Heat This Way
Aluminum’s heat capacity stems from lattice vibrations. Each atom oscillates within its crystal structure, storing kinetic energy as temperature rises. According to the Dulong-Petit law, metals typically exhibit ~25 J/mol·K molar heat capacity. Aluminum aligns closely at 24.2 J/mol·K—but why? Its face-centered cubic (FCC) lattice allows efficient phonon propagation, while low atomic mass (27 g/mol) enables rapid energy absorption. Crucially, deviations occur below 100K where quantum effects dominate. Above room temperature, however, aluminum’s heat capacity remains remarkably stable, varying less than 5% between 25–300°C (Source: NIST Chemistry WebBook, 2023). This consistency is a godsend for thermal engineers.
2. Measuring the Immeasurable: Experimental Insights
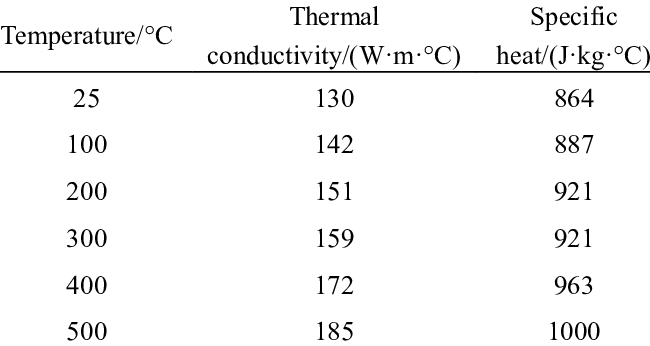
Quantifying heat capacity isn’t theoretical—it’s hands-on science. Differential Scanning Calorimetry (DSC) remains the gold standard. In my lab, we heat aluminum samples at controlled rates while measuring energy flux. For pure Al (1100 alloy), we consistently record 900 J/kg·K ±10 at 20°C. Alloying elements alter this: Copper increases it marginally (905 J/kg·K in 2024-T6), while silicon suppresses it (890 J/kg·K in 4047). Industry jargon like “Cp” (specific heat at constant pressure) matters here—ignoring it risks flawed thermal models. One pitfall? Assuming constant Cp. In reality, aluminum’s heat capacity rises 8% near its melting point (660°C) due to lattice anharmonicity.
3. Comparative Analysis: Aluminum vs. Structural Metals
| Material | Specific Heat (J/g·K) | Volumetric Heat Capacity (J/cm³·K) | Thermal Conductivity (W/m·K) |
|---|---|---|---|
| Aluminum (Pure) | 0.897 | 2.42 | 237 |
| Copper | 0.385 | 3.45 | 401 |
| Steel (Carbon) | 0.490 | 3.84 | 45 |
| Titanium | 0.523 | 2.36 | 22 |
| Magnesium | 1.02 | 1.77 | 156 |
Data sourced from ASM Handbook Vol. 2 (2022) and NIST Materials Database
Aluminum’s combination of low volumetric heat capacity and high conductivity explains why it heats rapidly but distributes energy efficiently—a critical advantage in heat sinks. Copper stores more heat per volume but weighs 3.3× more.
4. The Conductivity-Capacity Interplay
Heat capacity (Cp) and thermal conductivity (k) are often conflated—a grave error. Aluminum’s k=237 W/m·K enables quick heat transfer, while its Cp=897 J/kg·K governs how much energy it stores. This duality defines applications:
-
Low Cp + High k: Ideal for heat exchangers (rapid energy transfer without storage)
-
High Cp + Low k: Suitable for thermal buffers (e.g., cast iron cookware)
Aluminum’s balance makes it exceptional for transient thermal management. In electronics cooling, its low Cp allows quick responsiveness to chip temperature spikes.
5. Alloying Effects: Tailoring Thermal Response
Not all aluminums behave equally. Alloying elements disrupt lattice vibrations:
-
Copper (2xxx series): Increases Cp by 1-2% but reduces corrosion resistance
-
Silicon (4xxx series): Lowers Cp by 3-4%, enhancing fluidity for casting
-
Zinc (7xxx series): Negligible Cp change but boosts strength
Heat treatment also matters. Solutionized T4 state exhibits 2% higher Cp than artificially aged T6 due to solute atom dispersion (Source: Journal of Alloys and Compounds, Vol. 886, 2021).
6. Case Study: Satellite Thermal Management
Problem: A Low-Earth-Orbit satellite faced 200°C swings between sun exposure and shadow. Traditional materials caused thermal fatigue cracks.
Solution: We specified 6061-T6 aluminum for radiator panels. Its moderate Cp (897 J/kg·K) prevented overheating during insolation, while high emissivity (0.8+) facilitated radiative cooling.
Result: Temperature stabilized at 50±10°C, eliminating thermal stress failures. The Cp/k ratio proved optimal for cyclic environments.
7. Practical Applications Leveraging Heat Capacity
-
EV Battery Packs: Aluminum housings absorb heat during fast charging, delaying thermal runaway (Cp buffers temperature spikes)
-
Aircraft Skins: Low volumetric heat capacity minimizes fuel burn for cabin temperature control
-
Cookware: Thin aluminum pans heat rapidly (low Cp) but require constant energy input—unlike cast iron
-
Phase Change Materials (PCMs): Aluminum foam matrices enhance heat transfer in paraffin PCMs without degrading storage capacity
FAQ: Addressing Critical Questions
-
Q: Does aluminum’s heat capacity change with temperature?
A: Significantly. From 25°C to 600°C, Cp increases from 0.897 to 1.18 J/g·K due to intensified lattice vibrations. -
Q: How does anodizing affect thermal properties?
A: Anodized layers slightly reduce effective Cp (oxide Cp=0.8 J/g·K) but improve radiative heat dissipation. -
Q: Why does aluminum feel colder than steel at room temperature?
A: Despite lower Cp than steel (0.49 J/g·K), aluminum’s 5× higher conductivity pulls heat from your skin faster. -
Q: Can heat capacity predict melting behavior?
A: Indirectly. Aluminum’s Cp surge near 600°C signals latent heat absorption before phase change. -
Q: Is extruded vs. cast aluminum different thermally?
A: Microstructurally yes, but Cp differences are <1%. Conductivity varies more due to porosity.