What is the Density of Aluminum? The density of aluminum is 2.7 grams per cubic centimeter (g/cm³), or 2700 kilograms per cubic meter (kg/m³) at room temperature.
1. Why Density Matters in Materials Engineering
In materials engineering, density is more than just a number—it’s a critical property that determines how a material behaves in mechanical design, transportation, and thermal environments. When I’m choosing between metals like steel, copper, and aluminum, density is often the first parameter I examine. Why? Because it directly affects strength-to-weight ratio, thermal inertia, and cost-efficiency in transport.
2. Defining Density: Physical Meaning and Relevance
Density is the mass per unit volume of a substance. It is expressed as:
ρ = m / V
Where:
ρ = Density (kg/m³ or g/cm³)
m = Mass (kg or g)
V = Volume (m³ or cm³)
For aluminum, this value is 2.7 g/cm³, which is significantly lower than steel (~7.85 g/cm³) or copper (~8.96 g/cm³). This lower density makes aluminum ideal for applications requiring a high strength-to-weight ratio.
3. Aluminum Density at Different Temperatures and Alloys
Density can change slightly with temperature and alloy composition. Though pure aluminum has a density of 2.70 g/cm³, common aluminum alloys may vary from 2.65 to 2.85 g/cm³ depending on the alloying elements.
| Alloy Type | Common Designation | Density (g/cm³) |
|---|---|---|
| Pure Aluminum | 1xxx Series | 2.70 |
| Aluminum-Manganese | 3xxx Series | 2.73 |
| Aluminum-Silicon | Cast A356 | 2.68 |
| Aluminum-Zinc-Magnesium | 7xxx Series | 2.81 |
| Aluminum-Lithium | Al-Li Alloys | 2.63 |
Temperature also has a modest effect. According to the National Institute of Standards and Technology (NIST), aluminum’s density decreases slightly as temperature rises.
4. Comparison Table: Aluminum vs Other Metals
Here's how aluminum's density stacks up against other common industrial metals:
| Metal | Density (g/cm³) | Strength-to-Weight Ratio | Typical Use |
|---|---|---|---|
| Aluminum | 2.70 | High | Aerospace, automotive |
| Steel | 7.85 | Medium | Construction, tools |
| Copper | 8.96 | Low | Electrical wiring, plumbing |
| Titanium | 4.51 | Very High | Aerospace, medical implants |
| Magnesium | 1.74 | Moderate | Automotive, electronics |
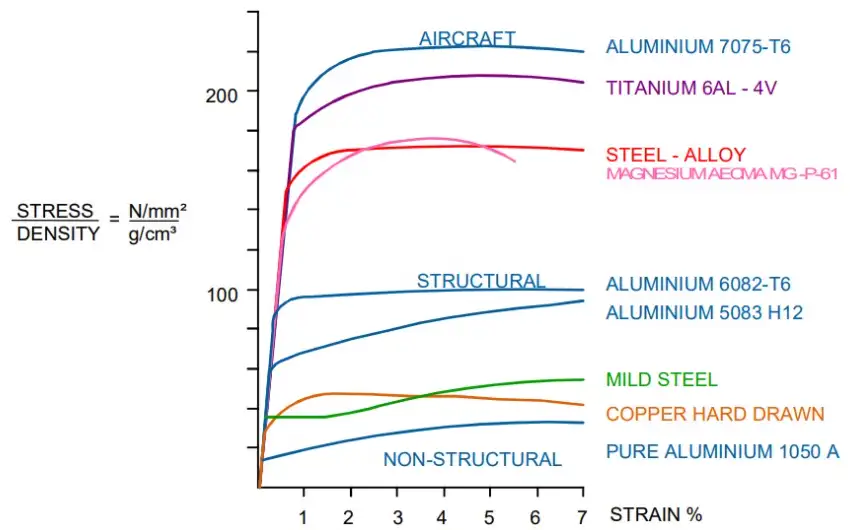
5. Case Study: How Aluminum's Density Reduces Aircraft Weight
Let’s look at the Boeing 737, which uses over 60,000 kg of aluminum in its structure.
Without Aluminum:
-
If built with steel instead (density 7.85 g/cm³), the aircraft weight would increase by approximately 120,000 kg, more than double.
Impacts:
-
Fuel consumption would increase by 35–50%.
-
Payload capacity would decrease.
-
Flight range would drop significantly.
This is why 95% of aircraft structures use aluminum alloys, primarily from the 2xxx and 7xxx series. [Source: ASM Aerospace Specification Metals Inc.]
6. Factors That Influence Aluminum's Apparent Density
While the theoretical density is 2.70 g/cm³, real-world measurements may vary due to:
-
Porosity in castings
-
Impurities like iron or silicon
-
Thermal expansion at elevated temperatures
-
Cold working or forging which may cause density variation due to lattice distortions
These variations are minor but important for precision engineering or aerospace-grade applications.
7. How to Accurately Measure Aluminum Density
The Archimedes’ principle is the most widely used method in industrial labs:
-
Weigh the sample in air
-
Weigh it again submerged in water
-
Calculate the volume based on displaced water
-
Use the formula ρ = m / V
Another method is using X-ray crystallography to derive lattice spacing and determine theoretical density, especially useful for alloys with unknown compositions.
8. Industrial Applications Driven by Aluminum’s Density
Because aluminum is so light, it has become indispensable in these fields:
Aerospace
Weight savings directly correlate with fuel efficiency.
Automotive
Electric vehicles like the Tesla Model S use aluminum body panels to extend range.
Construction
Curtain wall systems and cladding benefit from aluminum’s lightness and durability.
Electrical
While less conductive than copper, aluminum's light weight and cost savings make it a preferred material in high-voltage transmission lines.
9. Frequently Asked Questions (FAQs)
1. Is aluminum heavier or lighter than water?
Aluminum is heavier than water. Water has a density of 1.00 g/cm³, whereas aluminum is 2.70 g/cm³, so it sinks.
2. What is the density of aluminum foil?
Though very thin, aluminum foil still has the same density: 2.70 g/cm³. The difference lies in thickness, not material density.
3. How does aluminum compare to stainless steel in terms of density?
Stainless steel has a density of ~8.00 g/cm³, almost 3 times heavier than aluminum, making aluminum preferable when weight matters.
4. Does anodizing affect the density of aluminum?
Anodizing adds a thin oxide layer, but it doesn't significantly change the base metal’s density. However, it may slightly increase surface weight.
5. Can temperature changes alter aluminum’s density?
Yes, but only slightly. At 500°C, aluminum may lose about 2% of its density due to thermal expansion. For most practical applications, it’s negligible.